电话
021-50724187
The proto-oncogene c-fos belongs to the immediate early genes (IEGs) and is rapidly and transiently expressed in brain (Herrera and Robertson, 1996). It can be induced by various stimuli and is commonly used as a marker for neuronal activation (Morgan et al., 1987; Gaiddon et al., 1996; Chowdhury et al., 2000; Gallo et al., 2018; Wakhloo et al., 2020). Like many other IEGs c-Fos is a transcription factor. It binds as heterodimer with c-Jun to AP-1 binding sites (figure 1) and enhances the expression of many other genes involved in proliferation, differentiation, apoptosis or inflammation (Ye at al., 2014).
Figure 1: Illustration of the heterodimeric bZIP transcription factor c-Fos/c-Jun bound to DNA (adapted from Glover and Harrison, 1995 and PDB 1FOS)
Animal handling: While planning a c-Fos experiment with animals you should be aware of technical pitfalls affecting your research. For instance, you should consider that c-Fos expression is time dependent, susceptible for stress and can be detected after minutes (Herrera and Robertson, 1996). It is important to take into account that many factors like animal handling itself (e.g. needle injection) or the exposure to light during the dark phase (circadian activation) can influence c-Fos expression (Asanuma and Ogawa, 1994; Herrera and Robertson, 1996). Therefore, the experiment has to be planned and conducted accurately with appropriate control groups to avoid false positive results.
IHC protocol: Another point you should care about is your immunohistochemical (IHC) protocol. It seems that the total number of c-Fos positive cells is threshold dependent. Many parameters affect the detectable number of c-Fos positive cells: tissue storage conditions, method of sectioning (cryostat or vibratome), staining parameters (e.g. incubation temperature), signal enhancing reagents (Berghorn et al., 1994; Werner et al., 1996; Mayer and Bendayan, 2001) and, above all, primary antibody selection.
c-Fos antibody: Primary antibodies differ in their specificity, sensitivity and signal to noise ratio and these properties affect the outcome of your experiment. Therefore, we aim to provide highly specific and consistent c-Fos antibodies to support scientific research and experimental reproducibility. Since monoclonal antibodies have several advantages compared to polyclonal antibodies, we developed a new monoclonal recombinant rabbit anti-c-Fos antibody.
Antibodies share a common structure consisting of two heavy chains and two light chains (figure 2). Both heavy and light chains contain species-specific constant regions and variable sequences which are responsible for target recognition (Davies and Metzger, 1983).
Figure 2: Antibody scheme. Antibodies consist of two heavy and two light chains. Both heavy and light chains contain species-specific constant regions and variable sequences which are responsible for target recognition
In monoclonal antibody preparations originating from a defined hybridoma cell line, all individual antibody molecules are identical and share the same antigen recognition site. In contrast, polyclonal antibodies like the rabbit c-Fos antibody 226 003 consist of a mixture of antibody molecules that differ in their target recognition sequences. Once a serum is used up, a new animal has to be immunized. The obtained new serum contains a new mixture of c-Fos antibodies with new specificity and affinity properties, resulting in batch to batch variations concerning the experimental performance of the polyclonal c-Fos antibody.
With our monoclonal recombinant rabbit c-Fos antibody 226 008 we have permanently solved the problem of batch to batch variations. We sequenced the c-Fos target recognition domains of our consistently well performing rat monoclonal c-Fos antibody 226 017 and fused them to the constant antibody regions of a rabbit IgG (figure 3).
This highly defined monoclonal recombinant antibody can be expressed in mammalian cells under controlled conditions as an infinite resource with minimal batch to batch variations. It shows superior performance in our tested standard applications and is equivalent to or even better than our best rabbit polyclonal c-Fos batches (figure 4). While the polyclonal rabbit anti-c-Fos antibody 226 003 is very susceptible to changes in experimental conditions (for example, the incubation temperature in IHC experiments has an impact on signal intensity), the monoclonal recombinant rabbit anti-c-Fos antibody 226 008 leads to robust staining results in various experimental setups and is therefore the best choice for your experiments.
As time progresses, this monoclonal antibody will be used for many different applications and all the information gathered will contribute to a better characterization of this research reagent.
In contrast to polyclonal antibodies, this information accumulates and is never lost, making this research reagent an increasingly valuable and reliable tool in your hands.
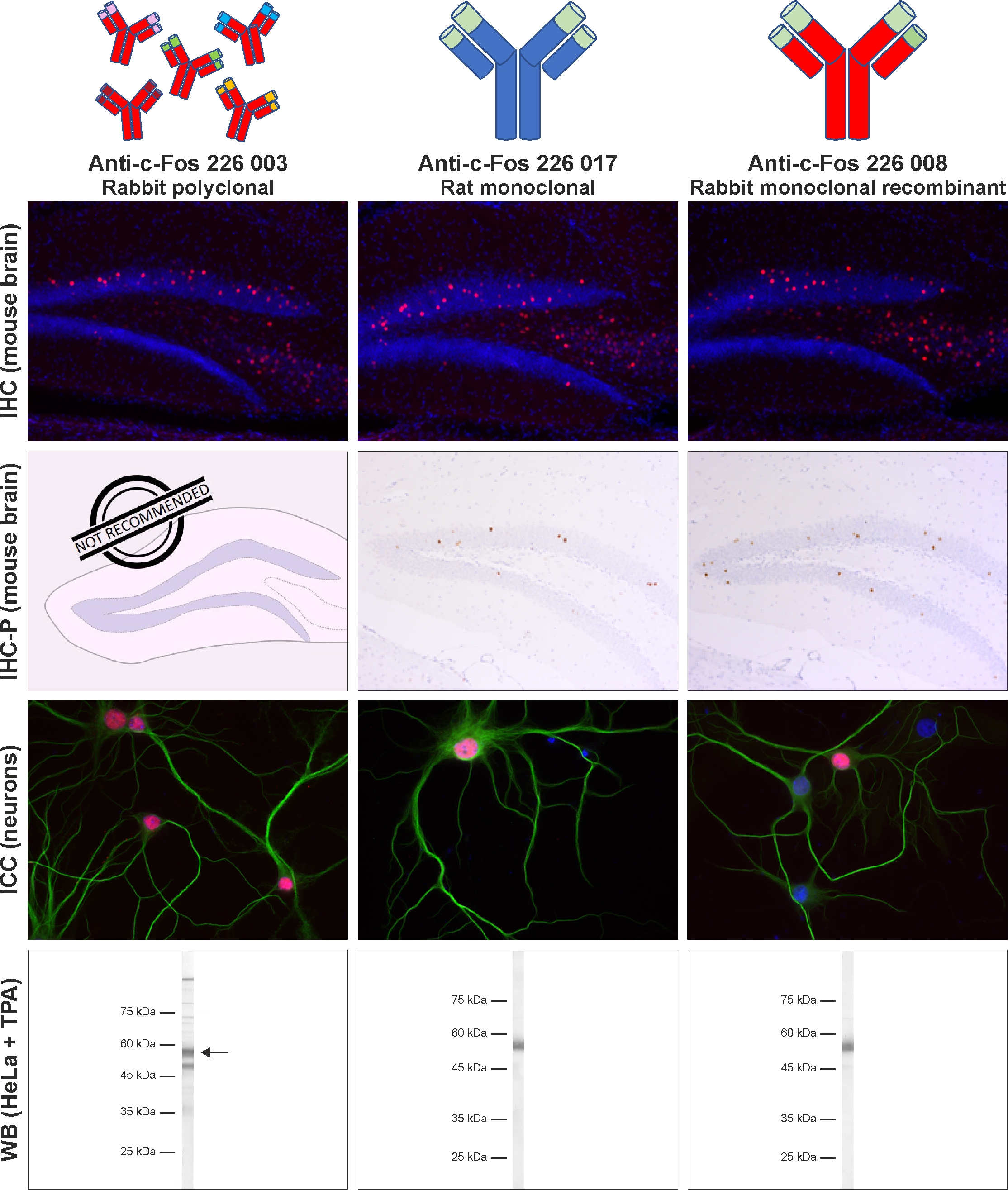
Figure 4: The monoclonal recombinant rabbit anti-c-Fos antibody 226 008 shows an excellent performance in all our standard applications. The recombinant rabbit anti-c-Fos antibody 226 008 was compared with the monoclonal rat anti-c-Fos antibody 226 017 and with the polyclonal rabbit anti-c-Fos antibody 226 003 under the same experimental conditions. In IHC and IHC-P experiments, c-Fos antibodies were utilized for indirect immunostaining of mouse and rat hippocampus sections (mouse sections shown; IHC: c-Fos = red, DAPI = blue; IHC-P: c-Fos = brown, haematoxylin = blue). In ICC experiments, c-Fos antibodies were used for indirect immunostaining of rat hippocampus neurons (c-Fos = red, MAP 2 188 011/188 002 = green, DAPI = blue). In western blot experiments, c-Fos was detected in nuclear extracts from TPA-stimulated HeLa cells (detection: AP-staining).

